Genetic Warfare: Targeting Tumors with Precision Gene Therapy Tools

Precision Gene Editing: The Rise of CRISPR and Beyond

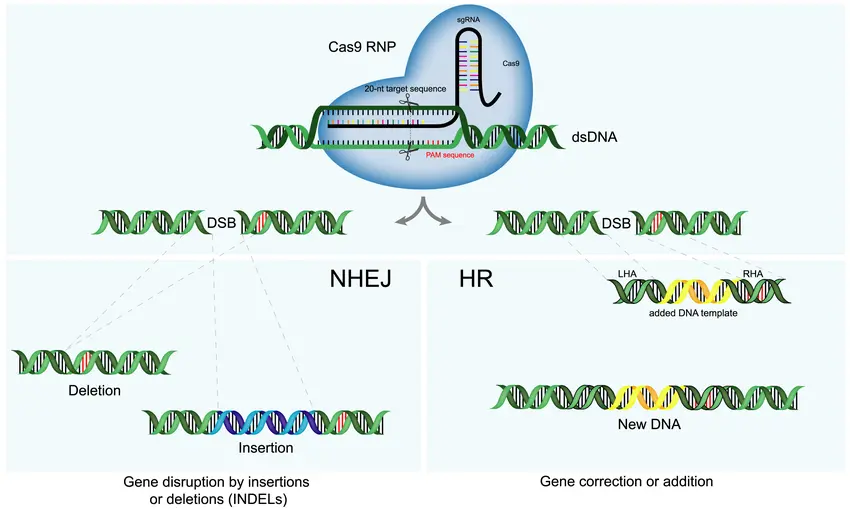
In the evolving landscape of biotechnology, precision gene editing stands out as one of the most powerful tools for understanding and treating human disease especially cancer. At the center of this revolution is CRISPR-Cas9, a genome-editing technology that has transformed research and therapeutic possibilities. But CRISPR is just the beginning. A new wave of gene-editing innovations is extending the frontiers of precision medicine even further.
CRISPR-Cas9: The Foundation of Modern Gene Editing
Discovered in bacterial immune systems and adapted for human cells, CRISPR-Cas9 allows scientists to cut DNA at precise locations, enabling the addition, deletion, or correction of genetic material. In cancer research and therapy, CRISPR is being used to:
- Knock out oncogenes that drive tumor formation
- Enhance immune cells, such as T cells, to recognize and kill cancer cells
- Model cancer mutations in lab settings for drug testing
Beyond CRISPR: Next-Generation Editing Technologies
While CRISPR-Cas9 is groundbreaking, it’s not without limitations such as the potential for off-target effects and reliance on DNA double-strand breaks. This has driven the development of more advanced tools, including:

Base Editors
These tools convert single DNA letters (A, C, G, T) without cutting the DNA strand. This is ideal for fixing point mutations, which account for many cancer-related genetic errors.

Prime Editing
Described as a “search and replace” function for DNA, prime editing can insert, delete, or rewrite sequences with high precision and fewer unintended effects.
CRISPR-Cas12 and Cas13
Cas12 is used for DNA, while Cas13 targets RNA. These enzymes offer new pathways for transient, non-permanent gene modulation, ideal for dynamic diseases like cancer.
Clinical Applications: Moving from Bench to Bedside
Gene editing is already making its way into clinical oncology. Current and upcoming applications include:
Gene-edited immune cell therapies for leukemia, lymphoma, and solid tumors
In vivo editing to silence cancer-causing genes directly in patient tissues
Synthetic lethality strategies, where gene editing is used to exploit vulnerabilities in tumor cells while sparing normal cells

Viral and Non-Viral Delivery Systems
As gene therapy advances from concept to clinical application, one critical factor determines its success: how therapeutic genetic material is delivered into target cells. Whether the goal is to insert, correct, silence, or replace genes, the delivery system must be safe, efficient, and targeted. This is especially vital in cancer therapy, where precision and safety are non-negotiable. Delivery systems are broadly divided into two categories: viral and non-viral, each with its own advantages, limitations, and emerging innovations.

CAR-T Cells and Beyond: Gene-Modified Immune Therapies
CAR-T Cell Therapy: A Breakthrough in Hematologic Cancers
Chimeric Antigen Receptor T cells (CAR-T) are created by extracting a patient’s T cells, modifying them to express a synthetic receptor (CAR) that targets a specific tumor antigen, and infusing them back into the patient.
Key successes include:
- CD19-targeted CAR-T for B-cell leukemia and lymphoma
- High response rates, even in relapsed or refractory cases
- Long-lasting remissions in a subset of patients
Despite its success in blood cancers, challenges remain for solid tumors, including:
- Limited infiltration into tumor sites
- Immunosuppressive tumor microenvironment
- Antigen heterogeneity leading to tumor escape
Next-Generation CAR-T Technologies
To address these limitations, researchers are developing advanced CAR-T platforms, such as

Dual or Tandem CAR-T Cells
Engineered to recognize two tumor antigens simultaneously, reducing the chance of immune escape.

Armored CAR-T Cells
Modified to secrete cytokines (like IL-12) or express dominant-negative receptors that help them resist immunosuppression.

Switchable CAR-T Systems
Incorporate "on/off" molecular switches to control activation and reduce toxicity, especially in solid tumors.

Universal Allogeneic CAR-T Cells
Produced from healthy donors and genetically edited to prevent graft-versus-host disease (GVHD), these “off-the-shelf” therapies offer scalable and faster options than autologous CAR-T.
Beyond CAR-T: Expanding the Gene-Modified Immune Arsenal
While CAR-T cells have led the way, other immune cell types are now being explored for gene modification and therapeutic use:
TCR-T Cell Therapy
Unlike CAR-T, T-cell receptor (TCR) therapies target intracellular antigens presented on MHC molecules, expanding the range of cancer targets (especially in solid tumors).
TCR therapies are highly specific to patient HLA types and tumor mutations, making them more personalized but also more complex.
Genetically Engineered Natural Killer (NK) Cells
NK cells are part of the innate immune system and can kill tumor cells without prior sensitization. Gene-edited NK therapies offer:
- Reduced cytokine release syndrome (CRS) risk
- Potential for allogeneic, off-the-shelf use
- Stronger activity when combined with cytokines or monoclonal antibodies
CAR-Macrophage Therapy (CAR-M)
A novel strategy where macrophages are engineered to recognize tumor antigens and stimulate innate and adaptive immunity.
CAR-Ms can reshape the tumor microenvironment and are being tested in solid cancers like breast and ovarian tumors.
Safety, Precision, and Control: The Role of Gene Editing
CRISPR-Cas9, TALENs, and base editors are now routinely used to enhance the safety and efficacy of gene-modified immune therapies. These tools enable:
- Knockout of inhibitory receptors (like PD-1)
- Insertion of synthetic genes for better control
- Removal of endogenous TCRs to reduce off-target activity and GVHD risk